Tandem Diabetes Care says the Food and Drug Administration (FDA) has cleared its smartphone app to program and cancel insulin doses from the t:slim X2 insulin pump. The company claims it's the first time the FDA has cleared a phone app for such a purpose.
The t:connect mobile app on iOS and Android will enable users to program and cancel bolus insulin doses from the pump, according to Tandem Diabetes Care. When connected to the pump, the app can display information about the last 24 hours of a user's glucose trends, changes in status (including alerts and alarms) and insulin therapy data.
Pumps typically require users to dial in insulin doses manually. Given that they are often able to view glucose readings on their handset, patients will be able to use their phone to determine how much insulin they need and then program their dose.
The FDA clearance could be a step toward a more convenient way for diabetes patients to administer insulin doses. Tandem Diabetes Care plans to offer the feature at no extra cost to new and in-warranty t:slim X2 insulin pump customers via a software update. The plan is to grant access to a limited number of users in the spring ahead of a broader rollout this summer.








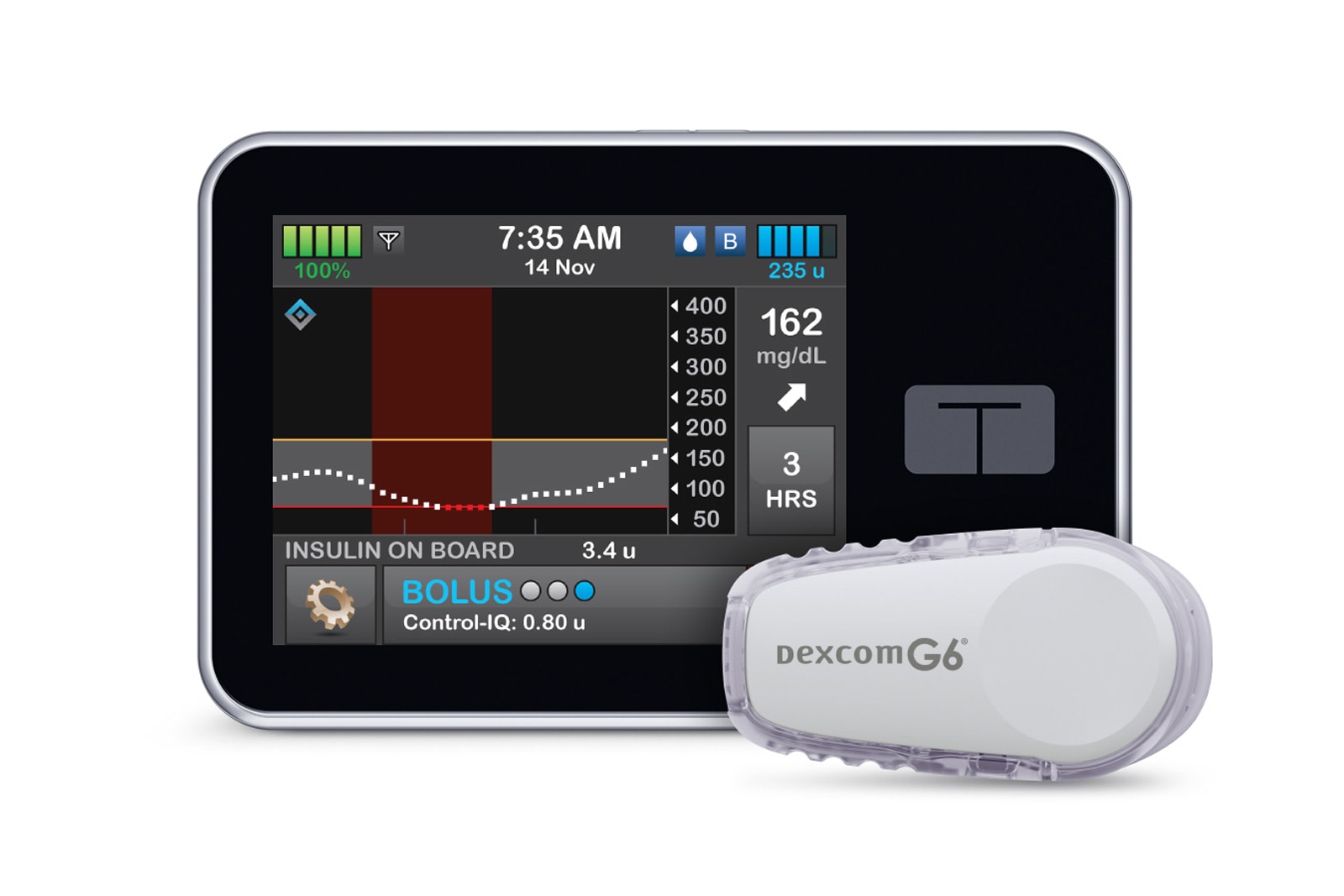 It might soon be decidedly easier for Americans to manage diabetes -- the FDA has approved the sale of an interoperable, automated insulin pump for the first time. Tandem Diabetes Care's updated t:slim X2 can pair with a Dexcom G6 glucose monitor to...
It might soon be decidedly easier for Americans to manage diabetes -- the FDA has approved the sale of an interoperable, automated insulin pump for the first time. Tandem Diabetes Care's updated t:slim X2 can pair with a Dexcom G6 glucose monitor to...